Treatment
Discover THE TREATMENT OPTIONS FOR lYME AND PANDAS
Treatment of Lyme Disease
Early Treatment
-
Many patients with early Lyme disease treated with short antibiotic courses (< 20 days) recover.
-
However, clinical experience and scientific literature both show that a significant proportion do not return to pre-Lyme health after such short courses.
ILADS Recommendations:
-
For patients with erythema migrans (EM):
-
4–6 weeks of doxycycline, amoxicillin, or cefuroxime
-
≥21 days of azithromycin is also acceptable (especially effective against European Borrelia strains).
-
-
For non-EM early disease: treatment is similar to EM protocols.
-
For disseminated disease: longer courses or antibiotic combinations may be required.
Treatment Response and Persistence
-
10–20% of patients remain ill following antibiotic therapy for early Lyme disease.
-
The clinical course and treatment response should guide further management.
-
Follow-up is essential:
-
If symptoms persist, recur, or new symptoms appear, persistent infection should be considered.
-
Borrelia burgdorferi has demonstrated the ability to survive antibiotics in laboratory, animal, and human studies.
-
Current testing cannot confirm eradication of the bacteria.
-
Chronic and Persistent Lyme Considerations
-
Persistent infection and co-infections must be considered in patients with ongoing symptoms.
-
Extended antibiotic therapy may be necessary when the risk of untreated infection outweighs the risks of long-term treatment.
-
Risks of long-term antibiotics exist but can be reduced with careful management.
-
Providers should engage in shared decision-making with patients:
-
Options for therapy
-
Risks and benefits of treatment
-
Risks of untreated persistent Lyme
-
Relapse vs. Re-Infection
-
Patients may be repeatedly infected with Lyme disease.
-
Both relapse and re-infection should be considered when symptoms recur after treatment.
Patient-Centered Care
ILADS emphasizes:
-
Person-specific, individualized care
-
Careful assessment and re-assessment of the full clinical picture
-
Treatment decisions guided by both the initial presentation and the course of illness if symptoms persist or return
Lyme disease
(Borrelia burgdorferi s.l.)
First-line (conventional) antibiotic therapy
Regimens below reflect the 2020 AAN/ACR/IDSA guideline and CDC clinical pages; tailor to age, pregnancy, allergies, and manifestations. IDSA+1CDC
Early localized (erythema migrans):
doxycycline 100 mg PO BID × 10 days; or amoxicillin 500 mg PO TID × 14 days; or cefuroxime axetil 500 mg PO BID × 14 days. Azithromycin only if others can’t be used. CDC
Early disseminated—cranial neuropathy (e.g., facial palsy): oral doxycycline × 14–21 days. CDC
Neuroborreliosis (meningitis/radiculoneuritis): doxycycline 200 mg/day PO or IV ceftriaxone 2 g QD × 14–21 days (choose route by severity/enteral tolerance). CDC
Carditis (atrioventricular block/myocarditis):
Outpatient, mild: oral agents (doxycycline/amoxicillin/cefuroxime/azithro) × 14–21 days.
Hospitalized or high-grade block: start IV ceftriaxone, switch to oral when improving; total 14–21 days. Temporary pacing is rarely needed; conduction usually recovers in days. IDSAOxford Academic
Lyme arthritis: oral doxycycline/amoxicillin/cefuroxime × 28 days; if minimal response, consider 2–4 weeks IV ceftriaxone or a second oral course, then transition to non-antibiotic arthritis management if synovitis persists despite adequate antibiotics. AAFP
Post-exposure prophylaxis (PEP)
For a high-risk Ixodes bite, consider single-dose doxycycline 200 mg (4.4 mg/kg up to 200 mg in children) within 72 hours of tick removal if criteria are met (Ixodes species, engorged, in a highly endemic area, etc.). CDC+1
Persistent symptoms after treatment (PTLDS)
Prolonged or repeated long-term antibiotics do not show durable benefit and carry harm in randomized trials; management is supportive and symptom-directed. New England Journal of Medicine
Common tick-borne co-infections (treat concurrently if suspected/confirmed)
-
Anaplasmosis (Anaplasma phagocytophilum): doxycycline (adults & children) 10–14 days (covers possible concurrent Lyme). CDC
-
Ehrlichiosis (Ehrlichia spp.): doxycycline for ≥5–7 days and ≥72 h fever-free with clinical improvement. CDC+1
-
Babesiosis (Babesia microti and others):
uncomplicated: atovaquone + azithromycin × 7–10 days; severe disease or intolerance: clindamycin + quinine; consider exchange transfusion for high parasitemia/severe hemolysis. Immunocompromised patients often need longer/more intensive therapy. IDSACDC -
Hard-tick relapsing fever (Borrelia miyamotoi): treat similarly to Lyme: ~14 days doxycycline (or amoxicillin); anticipate possible Jarisch–Herxheimer reaction. Evidence base is mostly case series. CDC
-
Bartonella henselae (cat-scratch disease; debated as an Ixodes co-pathogen): usually self-limited; azithromycin can hasten lymph node resolution; severe/disseminated disease requires specialist-guided combinations (e.g., doxycycline with rifampin), noting limited high-quality evidence. CDC
Clinical pearl: Fever with leukopenia and thrombocytopenia suggests anaplasmosis/ehrlichiosis; hemolytic anemia or parasitemia on smear suggests babesiosis. Co-infections can blunt response to standard Lyme therapy—treat all identified pathogens.
PANDAS/PANS
Definitions:
-
PANDAS = pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (abrupt-onset OCD/tics temporally linked to GAS).
-
PANS = broader, acute-onset OCD/restrictive eating with neuropsychiatric symptoms, infectious or non-infectious triggers.
Guideline landscape & controversy:
-
The PANS Research Consortium (PRC) published 2017 consensus treatment papers (Journal of Child & Adolescent Psychopharmacology):
Three pillars—
-
(1) treat triggers (often antimicrobials), (2) immunomodulation by severity,
-
(3) manage psychiatric/behavioral symptoms. Liebert Publications
-
The AAP 2025 Clinical Report provides a cautious, non-guideline overview and emphasizes limited evidence and careful differential diagnosis. Expect payer/policy variability and ongoing debate. AAP Publications
Conventional management (commonly accepted)
-
Treat the trigger/infection
-
GAS pharyngitis/sinusitis/skin infection: treat per standard pediatric guidelines (e.g., penicillin/amoxicillin; macrolide if true allergy). In severe, strep-driven, frequently relapsing cases, some experts consider secondary prophylaxis (practice varies; evidence low–moderate). PPN+1
-
Non-streptococcal triggers (e.g., Mycoplasma, influenza, SARS-CoV-2): manage per usual ID standards; the causal role is less certain.
-
-
Psychiatric/behavioral care for OCD/tics/anxiety
-
CBT/ERP is foundational; SSRIs and standard tic/OCD pharmacology as indicated, with careful titration (some children are medication-sensitive during flares). The Heartwood Program
-
-
Anti-inflammatory / immunomodulatory therapy
-
NSAIDs and short oral corticosteroid bursts are often used for flares.
-
IVIG (1–2 g/kg) or therapeutic plasma exchange (PLEX) may be considered for moderate–severe, function-threatening cases when there is a convincing immune-mediated presentation and inadequate response to the above. Evidence includes:
• a 1999 randomized trial showing symptom reductions with IVIG and PLEX in infection-triggered OCD/tics;
• a 2016 double-blind RCT of IVIG that did not meet its primary endpoint, though some secondary and open-label signals exist;
• newer observational reports with mixed results. Decisions are individualized, ideally in multidisciplinary care. PubMed+1PMC
-
Routine tonsillectomy is not recommended solely for PANDAS/PANS; consider only for standard ENT indications.
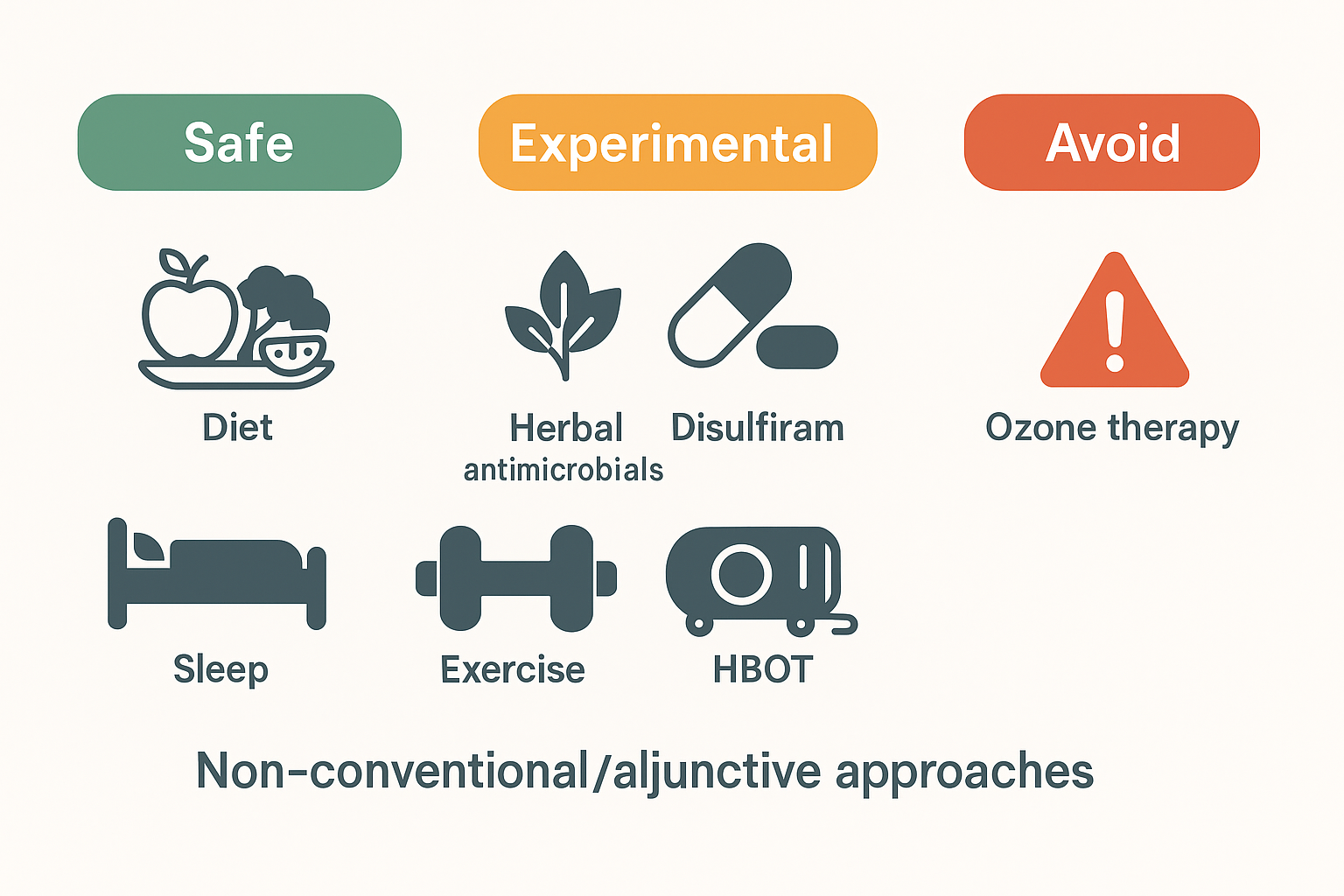
Non-conventional / adjunctive approaches (what we know)
Important framing: Many are unproven or have only in vitro or small, uncontrolled human data. Discuss risks, interactions, and cost with families; avoid substituting these for indicated antibiotics/immunotherapy.
-
Herbal/“botanical” antimicrobials (e.g., Cryptolepis sanguinolenta, Japanese knotweed/Polygonum cuspidatum, Artemisia annua): demonstrate in-vitro anti-Borrelia or anti-Babesia activity; robust clinical trials are lacking. Quality control and drug–herb interactions are concerns.
-
Disulfiram for persistent Lyme-attributed symptoms: mechanistic rationale and case series/pilot work exist; adverse effects can be significant; no definitive efficacy in large RCTs—use only with specialist oversight or in trials.
-
Hyperbaric oxygen therapy (HBOT): not guideline-recommended for Lyme; evidence limited to small, uncontrolled studies; reputable reviews highlight approved uses vs. unproven claims.
-
Ozone therapy and similar “oxidation” interventions: the FDA states ozone is a toxic gas with no proven safe medical application; avoid.
-
Diet, sleep, graded activity, micronutrient repletion: low risk and sensible as adjuncts to manage fatigue, sleep disturbance, pain, and mood—not disease-specific therapies.